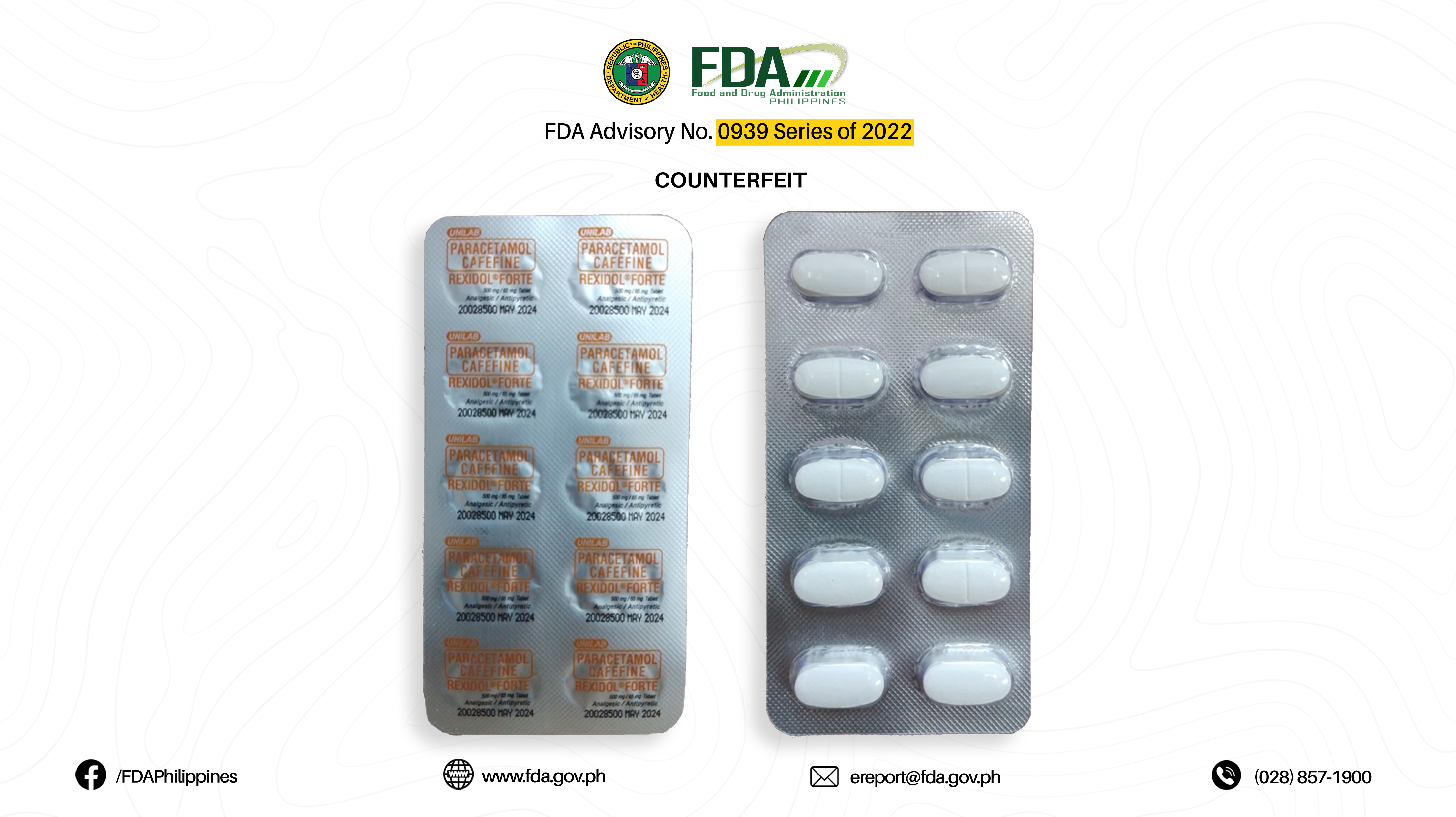
FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
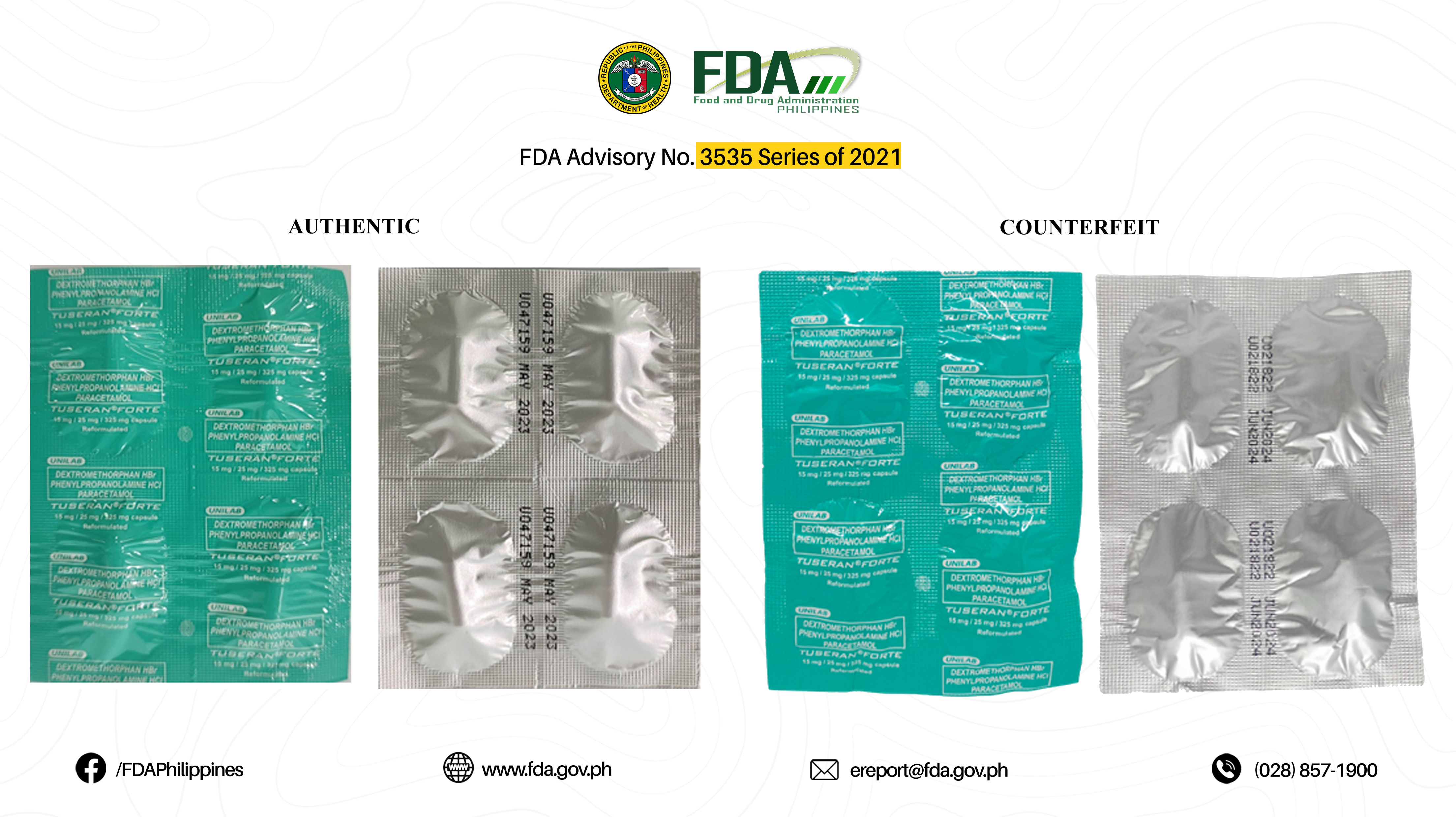
FDA Advisory No.2021-3535 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

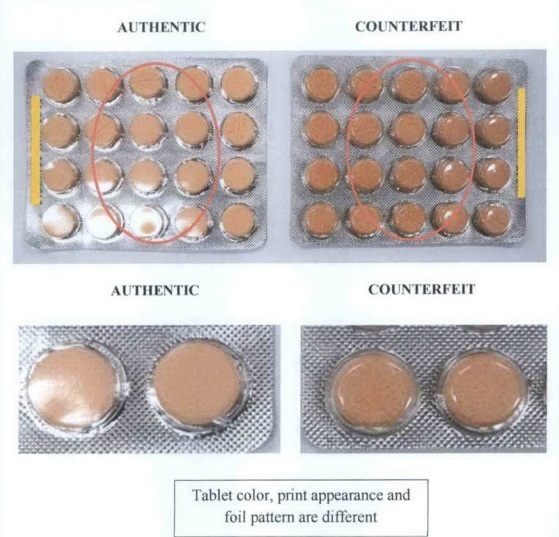
FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
FDA Advisory No. 2020-1348 || Public Health Warning Against the Purchase and Use of the following Counterfeit Drug Products: - Food and Drug Administration

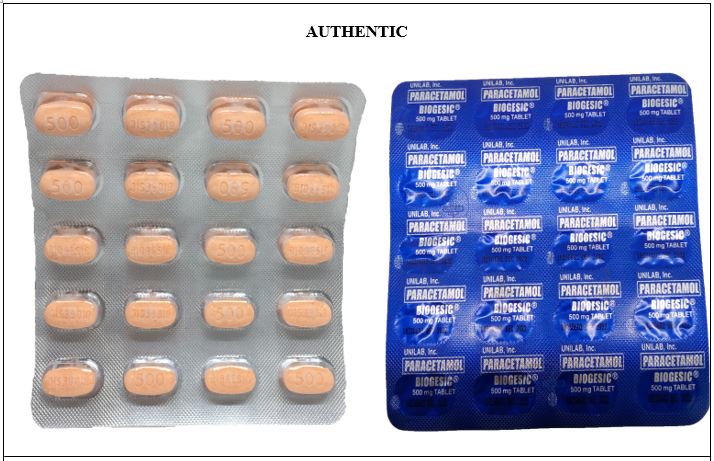
FDA Advisory No.2022-0135 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Paracetamol (Biogesic®) 500 mg Tablet” - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2021-1612 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product “Paracetamol Tablets USP 100mg” - Food and Drug Administration

FDA Advisory No.2022-0008 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product “Phenylpropanolamine HCl/ Chlorphenamine Maleate/ Paracetamol (Decolgen® Forte) 25 mg/ 2 mg/ 500 mg Tablet” -

FDA Advisory No.2022-0621 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2021-1723 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product “Perfalgan® Paracetamol 1 g in 100 mL Solution for Infusion 100 mL Vial” - Food and Drug Administration

FDA Advisory No.2022-0785 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Paracetamol (Biogesic®) 500 mg Tablet ” - Food and Drug Administration
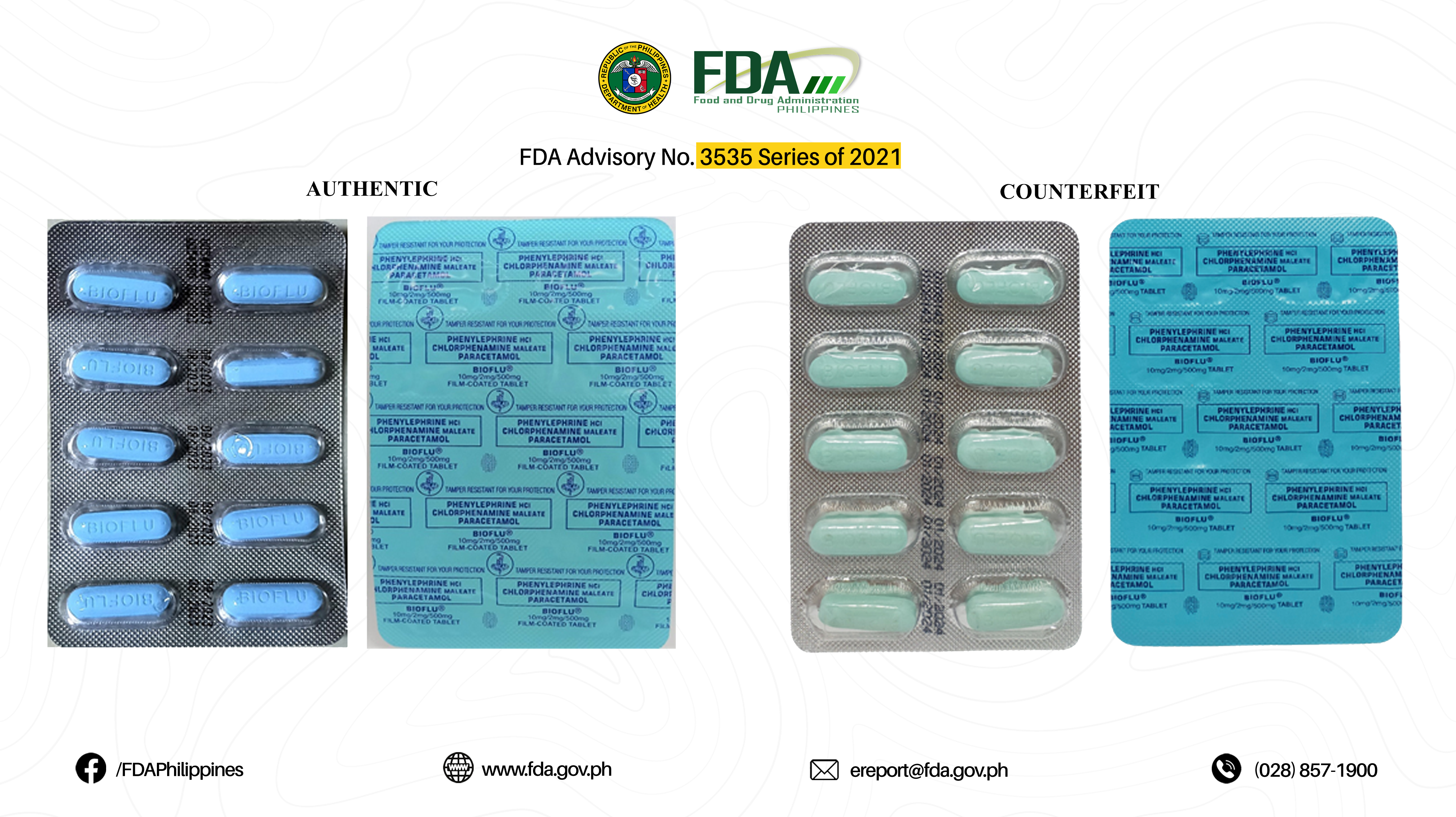
FDA Advisory No.2021-3535 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
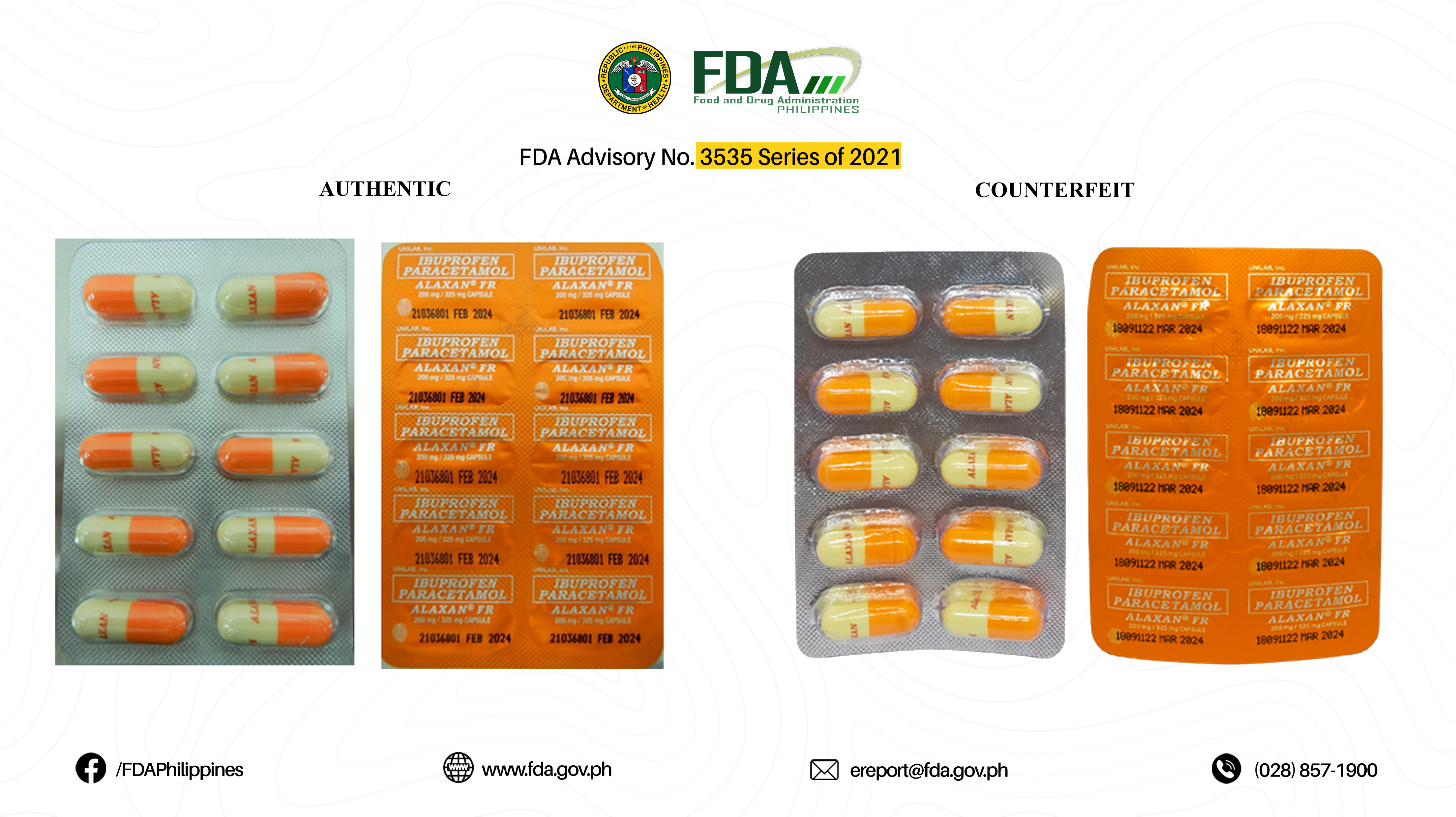
FDA Advisory No.2021-3535 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration