Liquid Biopsy to Identify Actionable Genomic Alterations | American Society of Clinical Oncology Educational Book

Blood-based liquid biopsies for prostate cancer: clinical opportunities and challenges | British Journal of Cancer

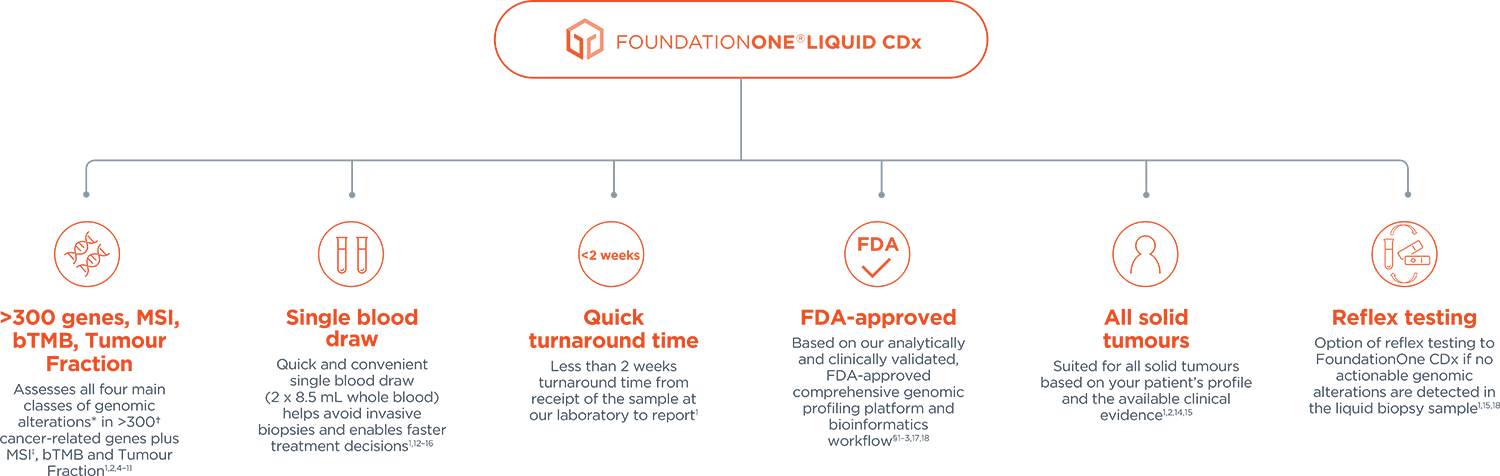
Foundation Medicine Introduces FoundationOne®Liquid, the Latest Advance in the Company's Liquid Biopsy Test for Solid Tumors in Patients with Advanced Cancer | Business Wire

Clinical and pathological features associated with circulating tumor DNA content in real‐world patients with metastatic prostate cancer - Antonarakis - 2022 - The Prostate - Wiley Online Library

![Foundation Medicine review - 7 facts you should know [DECEMBER 2021] Foundation Medicine review - 7 facts you should know [DECEMBER 2021]](https://nebula.org/blog/wp-content/uploads/2021/12/FoundationOne-CDx-testing-kit.png)