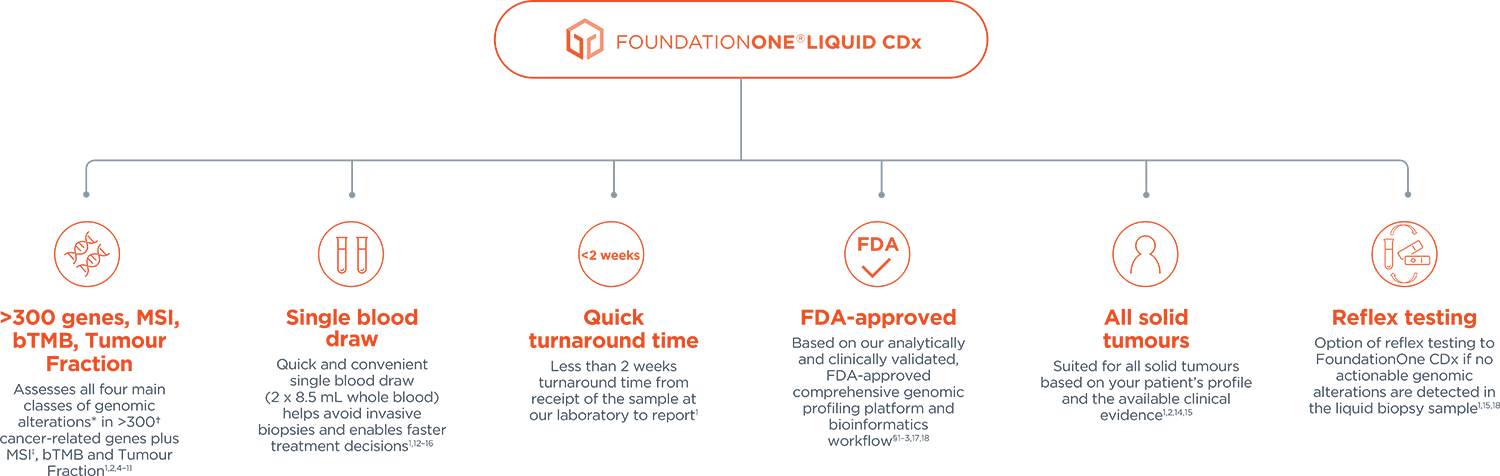
FoundationOne®CDx Technical Information Foundation Medicine, Inc. 150 Second Street, Cambridge, MA 02141 Phone: 617.418.2200 I

Comprehensive genomic profiling in oncology – from vision to reality | Latest news for Doctors, Nurses and Pharmacists | Pharmacy

Foundation Medicine on LinkedIn: Join Foundation Medicine's Lucas Dennis, VP of Franchise Development, for…

Foundation Medicine and Chugai Announce Partnership with National Cancer Center for the Use of FoundationOne®Liquid in the Third Stage of SCRUM-Japan | Business Wire
Roche France, Foundation Medicine and the Institute Gustave Roussy announce unique partnership to provide in-house liquid biopsy genomic testing to cancer patients in France | Gustave Roussy

Foundation Medicine Acquires Lexent Bio, Inc., to Accelerate Liquid Biopsy Research and Development, and Advance Cancer Care

Foundation Medicine Introduces FoundationOne®Liquid, the Latest Advance in the Company's Liquid Biopsy Test for Solid Tumors in Patients with Advanced Cancer | Technology Networks
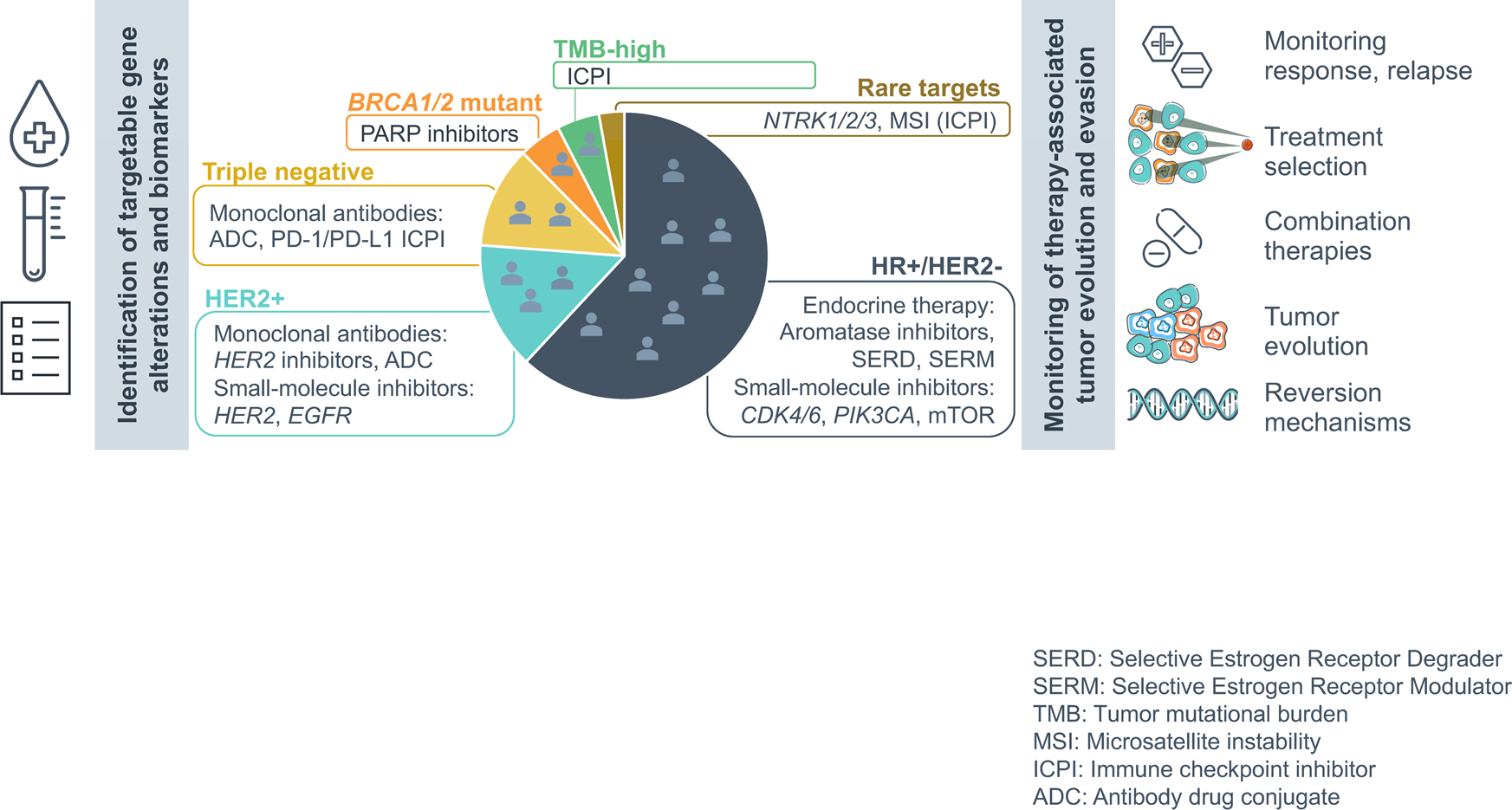
Tissue and liquid biopsy profiling reveal convergent tumor evolution and therapy evasion in breast cancer | Nature Communications